Midhuna's Science Corner
Friday, April 26, 2019
Thursday, April 18, 2019
Body composition - Chemistry in our everyday life
Body Composition
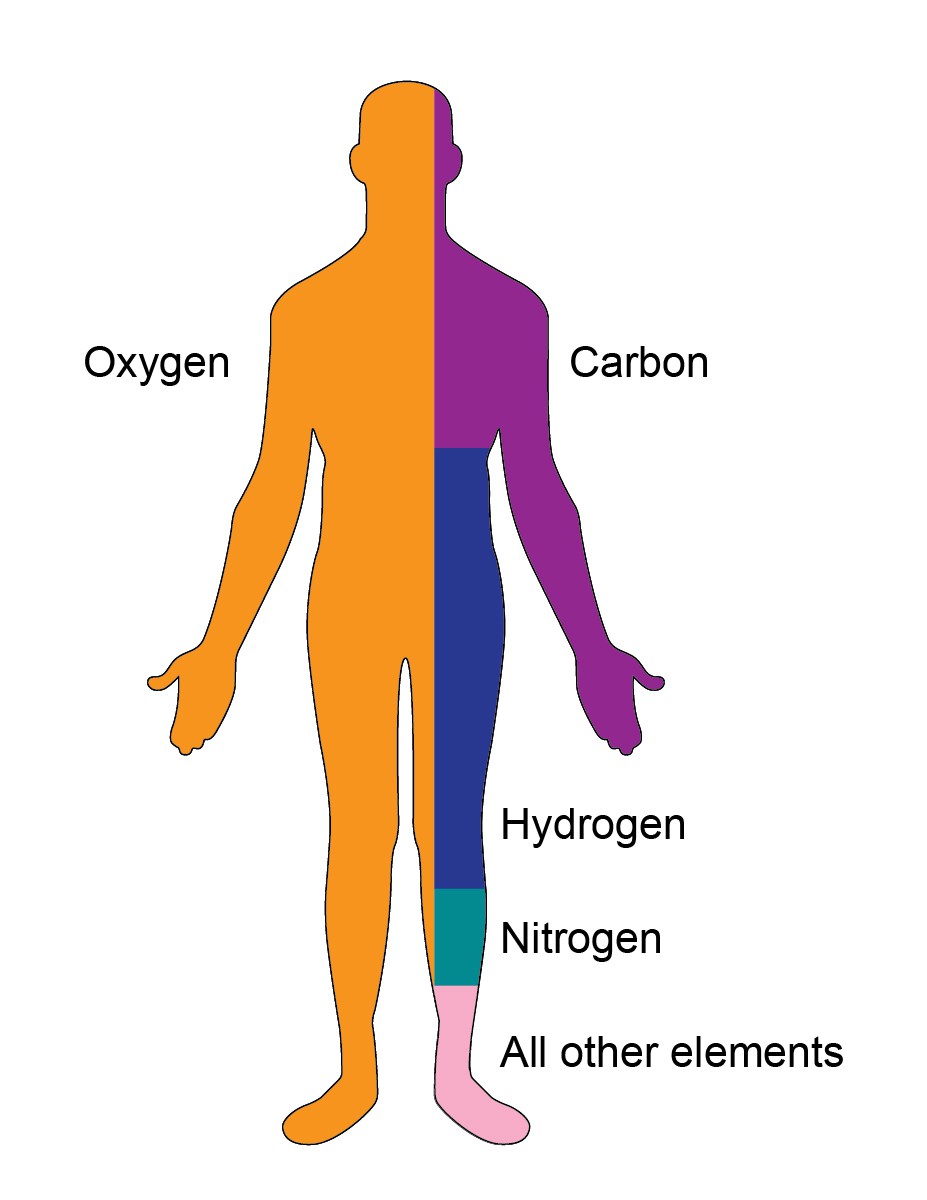
Your body is a fascinating place. Carbon and oxygen are the two most essential elements of the body. The other elements which are present in your body are nitrogen, phosphorous, hydrogen, oxygen, calcium, potassium, sulfur, magnesium, etc.
| ELEMENT | PERCENTAGE IN THE BODY | FUNCTION |
|---|---|---|
| Oxygen (O) | 65 | • Primary solvent • Regulate temperature & osmotic pressure |
| Carbon (C) | 18 | • Energy source • Building blocks of the body |
| Hydrogen (H) | 10 | • Present in water and all organic molecules |
| Nitrogen (N) | 3 | • Found in proteins and nucleic acids |
| Calcium (Ca) | 1.5 | • Critical for muscle contraction |
| Phosphorous (P) | 1.0 | • Acts as a buffer • Provides strength and structure to bones and teeth |
| Potassium (K) | 0.35 | • Crucial electrolyte • Helps in transmission of nerve impulse • Regulates heartbeat |
| Sulfur (S) | 0.25 | • Renders shape to the proteins which aid in proper functioning of proteins |
| Sodium (Na) | 0.15 | • Important electrolyte for regulating the amount of water • Helps in nerve signaling |
| Magnesium (Mg) | 0.05 | • Required in more than 300 biochemical reactions • Builds muscle and bones • Chief cofactor in many enzymatic reactions |
| Iron (Fe) | 0.006 | • Help in blood production |
| Copper (Cu), Zinc (Zn), Selenium (Se), Molybdenum (Mb), Fluorine (F), Iodine (I), Manganese (Mn), Cobalt (Co) | Total is less than 0.70 | • Copper is a micronutrient for the growth and development, and also essential for various metabolic functions • Zinc plays an important role in cell growth, cell division, wound healing, and the breakdown of carbohydrates • Selenium protects the body from oxidative damage • Molybdenum removes toxins from metabolism of sulfur containing amino acids • Fluorine is responsible for mineralization and formation of tooth enamel • Iodine is essential for formation of thyroid hormones • Manganese helps in the formation of connective tissues, bones, blood-clotting factors, sex hormones in addition to being critical in fat and carbohydrate metabolism, calcium absorption, and blood sugar regulation |
| Lithium (Li), Strontium (Sr), Aluminium (Al), Silicon (Si), Lead (Pb), Arsenic (As), Vanadium (V), Bromine (Br) | Present in trace amounts | • Lithium is essential for maintaining neurological health • Strontium aids in bone formation and prevent bone loss; the radioactive form of strontium can also kill some cancer cells • Aluminum is responsible for chromatin compaction • Silicon helps in promoting firmness and strength in arteries, connective tissues, tendons, skin, and eyes • Vanadium plays a role in metabolizing enzymes |
Wednesday, April 10, 2019
Light - Relation between speed and source
The speed of light doesn't change when you boost your light source. Imagine throwing a ball as fast as you can. Depending on what sport you're playing, you might get all the way up to 100 miles per hour (~45 meters/second) using your hand-and-arm alone. Now, imagine you're on a train (or in a plane) moving incredibly quickly: 300 miles per hour (~134 m/s). If you throw the ball from the train, moving in the same direction, how fast does the ball move? You simply add the speeds up: 400 miles per hour, and that's your answer. Now, imagine that instead of throwing a ball, you emit a beam of light instead. Add the speed of the light to the speed of the train... and you get an answer that's completely wrong.
Really, you do! This was the central idea of Einstein's theory of special relativity, but it wasn't Einstein who made this experimental discovery; it was Albert Michelson, who's pioneering work in the 1880s demonstrated that this was the case. Whether you fired a beam of light in the same direction that Earth moved, perpendicular to that direction, or antiparallel to that direction made no difference. Light always moved at the same speed: c, the speed of light in vacuum. Michelson developed his interferometer to measure the motion of the Earth through the aether, and instead paved the way for relativity. His 1907 Nobel prize remains the world's most famous null result, and the most important one in scientific history.
Tuesday, April 2, 2019
Plum pudding model of an atom
99.9% of an atom's mass is concentrated in an incredibly dense nucleus. Have you ever heard of the 'plum pudding' model of the atom? It sounds quaint today, but it was generally accepted at the start of the 20th century that atoms were made of a mix of negatively charged electrons (behaving like plums) embedded in a positively-charged medium (which behaved like pudding) that filled all of space. Electrons could be stripped off or stolen, explaining the phenomenon of static electricity. For years, J.J. Thomson's model of a composite atom, with small electrons in a positively charged substrate, was generally accepted. Until, that is, it was put to the test by Ernest Rutherford.
By firing high-energy, charged particles (from radioactive decays) at a very thin sheet of gold foil, Rutherford fully expected that all the particles would pass through. And most of them did, but a few spectacularly bounced back! As Rutherford recounted:
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.
What Rutherford discovered was the atomic nucleus, containing virtually all the mass of an atom, confined to a volume one-quadrillionth (10-15) the size of the entire thing. It was the birth of modern physics, and it paved the way for the quantum revolution of the 20th century.
Friday, March 29, 2019
Saturday, March 23, 2019
Cosmic microwave background -Origin of universe
The Universe began with a bang, but that discovery was a complete accident. In the 1940s, George Gamow and his collaborators put forth a radical idea: that the Universe that was expanding and cooling today was not only hotter and denser in the past, but arbitrarily so. If you extrapolated back far enough, you'd have a Universe hot enough to ionize all the matter in it, while even farther back you'd break apart atomic nuclei. The idea became known as the Big Bang, with two major predictions arising:
- The Universe we began with wouldn't have only matter made of mere protons and electrons, but would consist of a mix of the light elements, fused together in the high-energy, early Universe.
- When the Universe cooled enough to form neutral atoms, that high-energy radiation would be released, and would travel in a straight line for all eternity until it collided with something, red shifting and losing energy as the Universe expanded.
This "cosmic microwave background" was predicted to be just a few degrees above absolute zero.
In 1964, Arno Penzias and Bob Wilson accidentally discovered the Big Bang's leftover glow. Working with a radio antenna at Bell Labs to study radar, they found uniform noise everywhere they looked on the sky. It wasn't the Sun, or the galaxy, or Earth's atmosphere... but they didn't know what it was. So they cleaned out the inside of the antenna with mops, removing pigeons in the process, but still the noise persisted. It was only when the results were shown to a physicist familiar with the Princeton group's (Dicke, Peebles, Wilkinson, etc.) detailed predictions, and with the radiometer they were building to detect exactly this type of signal, that they recognized the significance of what they found. For the first time, the origin of our Universe was known.
Wednesday, March 13, 2019
Minuscle - the nearly-invisible particle
'Missing energy' leads to the discovery of a minuscule, nearly-invisible particle. In all the interactions we've ever seen between particles, energy is always conserved. It can be transformed from one type into another — potential, kinetic, rest mass, chemical, atomic, electrical, etc. — but it can never be created nor destroyed. Which is why it was so puzzling, nearly a century ago, when it was found that some radioactive decays have slightly less total energy in their products than in the initial reactants. It led Bohr to postulate that energy was always conserved... except for when it was lost. But Bohr was mistaken, and it was Pauli who had other ideas.
Pauli contended that energy must be conserved, and so way back in 1930, he proposed a new particle: the neutrino. This "little neutral one" would not interact electromagnetically, but would instead have a minuscule mass and carry kinetic energy away. While many were skeptical, experiments from the products of nuclear reactions eventually detected both neutrinos and antineutrinos in the 1950s and 1960s, which helped lead physicists to both the Standard Model and the model of the weak nuclear interactions. It's a stunning example of how theoretical predictions can sometimes lead to a spectacular advance, once the proper experimental techniques are developed.
Pauli contended that energy must be conserved, and so way back in 1930, he proposed a new particle: the neutrino. This "little neutral one" would not interact electromagnetically, but would instead have a minuscule mass and carry kinetic energy away. While many were skeptical, experiments from the products of nuclear reactions eventually detected both neutrinos and antineutrinos in the 1950s and 1960s, which helped lead physicists to both the Standard Model and the model of the weak nuclear interactions. It's a stunning example of how theoretical predictions can sometimes lead to a spectacular advance, once the proper experimental techniques are developed.
Monday, March 4, 2019
High energy particles - the science behind
All the particles we interact with have high-energy, unstable cousins. It's often said that advances in science aren't met with "eureka!" but rather with "that's funny," but this actually happened in fundamental physics! If you charge up an electroscope — where two conducting metal leaves are connected to another conductor — both leaves will gain the same electric charge, and repel one another as a result. If you place that electroscope in a vacuum, the leaves shouldn't discharge, but over time, they do. The best idea we had for this discharge was that there were high-energy particles hitting Earth from outer space, cosmic rays, and the products of these collisions were discharging the electroscope.
In 1912, Victor Hess conducted balloon-borne experiments to search for these high-energy cosmic particles, discovering them immediately in great abundance and becoming the father of cosmic rays. By constructing a detection chamber with a magnetic field in them, you could measure both the velocity and charge-to-mass ratio based on how the particle’s track curves. Protons, electrons, and even the first particles of antimatter were detected via this method, but the biggest surprise came in 1933, when Paul Kunze, working with cosmic rays, discovered a track from a particle that was just like the electron... except hundreds of times heavier!
The muon, with a lifetime of just 2.2 microseconds, was later experimentally confirmed and detected by Carl Anderson and his student, Seth Neddermeyer, using a cloud chamber on the ground. When the physicist I.I. Rabi, himself a Nobel Laureate for the discovery of nuclear magnetic resonance, learned of the muon's existence, he famously quipped, "Who ordered that?" It was later discovered that both composite particles (like the proton and neutron) and fundamental ones (quarks, electrons, and neutrinos) all have multiple generations of heavier relatives, with the muon being the first "generation 2" particle ever discovered.
In 1912, Victor Hess conducted balloon-borne experiments to search for these high-energy cosmic particles, discovering them immediately in great abundance and becoming the father of cosmic rays. By constructing a detection chamber with a magnetic field in them, you could measure both the velocity and charge-to-mass ratio based on how the particle’s track curves. Protons, electrons, and even the first particles of antimatter were detected via this method, but the biggest surprise came in 1933, when Paul Kunze, working with cosmic rays, discovered a track from a particle that was just like the electron... except hundreds of times heavier!
The muon, with a lifetime of just 2.2 microseconds, was later experimentally confirmed and detected by Carl Anderson and his student, Seth Neddermeyer, using a cloud chamber on the ground. When the physicist I.I. Rabi, himself a Nobel Laureate for the discovery of nuclear magnetic resonance, learned of the muon's existence, he famously quipped, "Who ordered that?" It was later discovered that both composite particles (like the proton and neutron) and fundamental ones (quarks, electrons, and neutrinos) all have multiple generations of heavier relatives, with the muon being the first "generation 2" particle ever discovered.
Tuesday, February 26, 2019
Wednesday, February 20, 2019
Friday, February 15, 2019
EPC3- Ex 9: Video Conferencing
Video conferencing is a visual communication session between two or more users regardless of their location, featuring audio and video content transmission in real time.
Video Conferencing Benefits
Save your time. You can run video conferences with remote colleagues on the run right from your desktop or meeting room. With video meeting you don’t waste your time and money on business vacations, cut event management costs, etc.
Easy-to-use. You just need to schedule your video meeting, invite your colleagues and start video conference right away! Your video conferencing system will also send you a notification to remind you of your meeting. Additionally, video conferencing system interface is extremely user-friendly and does not need additional trainings.
Collaboration tools. For efficient workflow video conferencing systems often provide different collaboration tools, such as screen and content sharing, slideshow, instant messaging. Collaboration tools allow multiple teams to work on a joint project, share their results and brainstorm ideas.
Real-life impressions. As compared to phone talks, video conferencing is much closer to real life as it features visual contact. During a video meeting you can see user’s emotions and articulation and establish eye contact, which is crucial for social communication. Additionally, video conferencing systems do not allow users to distract, making it easier to focus on the communications as during a real meeting.
Security. Modern video conferencing systems are based on specialized codecs, proprietary protocols and actively using encryption, which is why security risks can only be caused by a human factor.
Subscribe to:
Comments (Atom)
-
'Missing energy' leads to the discovery of a minuscule, nearly-invisible particle . In all the interactions we've ever seen bet...
-
The Universe began with a bang, but that discovery was a complete accident . In the 1940s, George Gamow and his collaborators put forth a ...