Friday, April 26, 2019
Thursday, April 18, 2019
Body composition - Chemistry in our everyday life
Body Composition
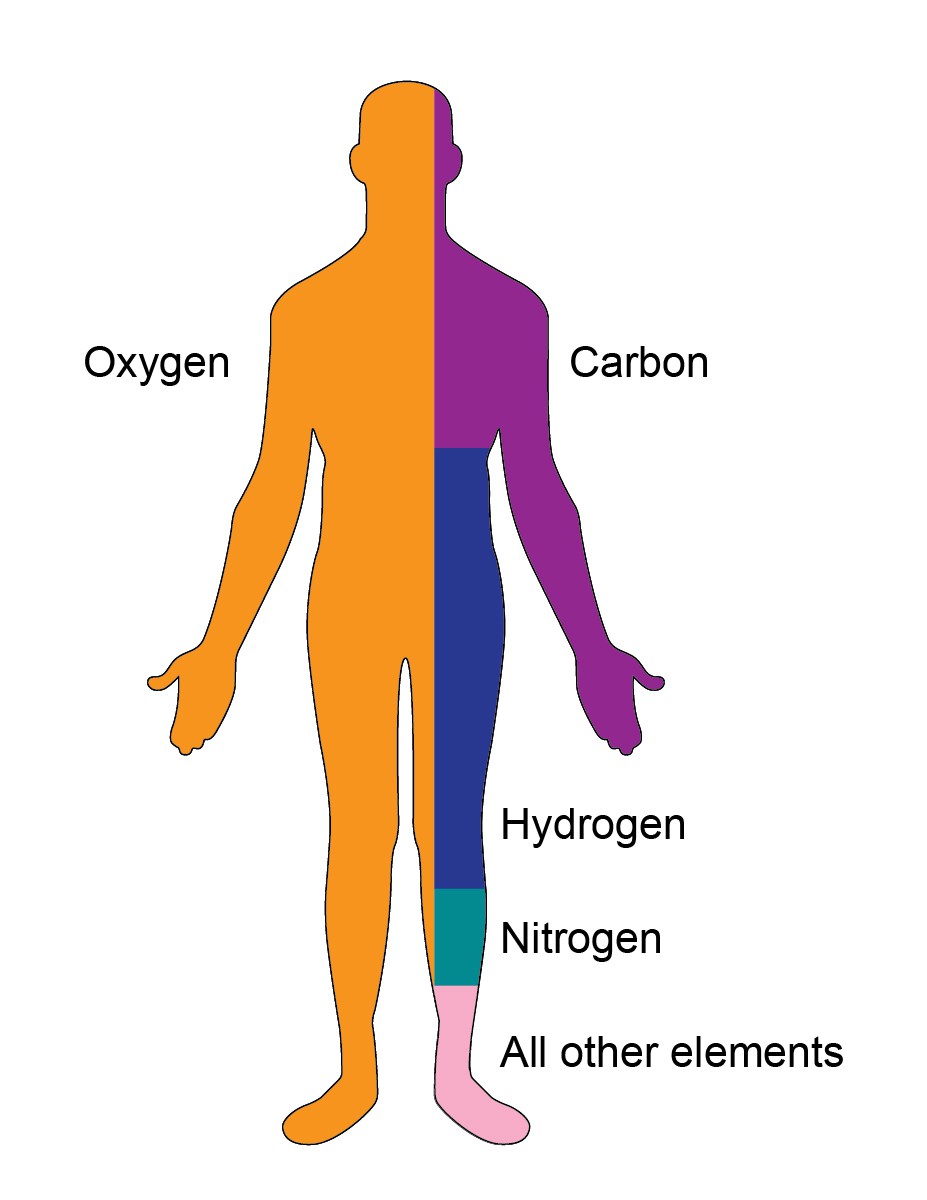
Your body is a fascinating place. Carbon and oxygen are the two most essential elements of the body. The other elements which are present in your body are nitrogen, phosphorous, hydrogen, oxygen, calcium, potassium, sulfur, magnesium, etc.
| ELEMENT | PERCENTAGE IN THE BODY | FUNCTION |
|---|---|---|
| Oxygen (O) | 65 | • Primary solvent • Regulate temperature & osmotic pressure |
| Carbon (C) | 18 | • Energy source • Building blocks of the body |
| Hydrogen (H) | 10 | • Present in water and all organic molecules |
| Nitrogen (N) | 3 | • Found in proteins and nucleic acids |
| Calcium (Ca) | 1.5 | • Critical for muscle contraction |
| Phosphorous (P) | 1.0 | • Acts as a buffer • Provides strength and structure to bones and teeth |
| Potassium (K) | 0.35 | • Crucial electrolyte • Helps in transmission of nerve impulse • Regulates heartbeat |
| Sulfur (S) | 0.25 | • Renders shape to the proteins which aid in proper functioning of proteins |
| Sodium (Na) | 0.15 | • Important electrolyte for regulating the amount of water • Helps in nerve signaling |
| Magnesium (Mg) | 0.05 | • Required in more than 300 biochemical reactions • Builds muscle and bones • Chief cofactor in many enzymatic reactions |
| Iron (Fe) | 0.006 | • Help in blood production |
| Copper (Cu), Zinc (Zn), Selenium (Se), Molybdenum (Mb), Fluorine (F), Iodine (I), Manganese (Mn), Cobalt (Co) | Total is less than 0.70 | • Copper is a micronutrient for the growth and development, and also essential for various metabolic functions • Zinc plays an important role in cell growth, cell division, wound healing, and the breakdown of carbohydrates • Selenium protects the body from oxidative damage • Molybdenum removes toxins from metabolism of sulfur containing amino acids • Fluorine is responsible for mineralization and formation of tooth enamel • Iodine is essential for formation of thyroid hormones • Manganese helps in the formation of connective tissues, bones, blood-clotting factors, sex hormones in addition to being critical in fat and carbohydrate metabolism, calcium absorption, and blood sugar regulation |
| Lithium (Li), Strontium (Sr), Aluminium (Al), Silicon (Si), Lead (Pb), Arsenic (As), Vanadium (V), Bromine (Br) | Present in trace amounts | • Lithium is essential for maintaining neurological health • Strontium aids in bone formation and prevent bone loss; the radioactive form of strontium can also kill some cancer cells • Aluminum is responsible for chromatin compaction • Silicon helps in promoting firmness and strength in arteries, connective tissues, tendons, skin, and eyes • Vanadium plays a role in metabolizing enzymes |
Wednesday, April 10, 2019
Light - Relation between speed and source
The speed of light doesn't change when you boost your light source. Imagine throwing a ball as fast as you can. Depending on what sport you're playing, you might get all the way up to 100 miles per hour (~45 meters/second) using your hand-and-arm alone. Now, imagine you're on a train (or in a plane) moving incredibly quickly: 300 miles per hour (~134 m/s). If you throw the ball from the train, moving in the same direction, how fast does the ball move? You simply add the speeds up: 400 miles per hour, and that's your answer. Now, imagine that instead of throwing a ball, you emit a beam of light instead. Add the speed of the light to the speed of the train... and you get an answer that's completely wrong.
Really, you do! This was the central idea of Einstein's theory of special relativity, but it wasn't Einstein who made this experimental discovery; it was Albert Michelson, who's pioneering work in the 1880s demonstrated that this was the case. Whether you fired a beam of light in the same direction that Earth moved, perpendicular to that direction, or antiparallel to that direction made no difference. Light always moved at the same speed: c, the speed of light in vacuum. Michelson developed his interferometer to measure the motion of the Earth through the aether, and instead paved the way for relativity. His 1907 Nobel prize remains the world's most famous null result, and the most important one in scientific history.
Tuesday, April 2, 2019
Plum pudding model of an atom
99.9% of an atom's mass is concentrated in an incredibly dense nucleus. Have you ever heard of the 'plum pudding' model of the atom? It sounds quaint today, but it was generally accepted at the start of the 20th century that atoms were made of a mix of negatively charged electrons (behaving like plums) embedded in a positively-charged medium (which behaved like pudding) that filled all of space. Electrons could be stripped off or stolen, explaining the phenomenon of static electricity. For years, J.J. Thomson's model of a composite atom, with small electrons in a positively charged substrate, was generally accepted. Until, that is, it was put to the test by Ernest Rutherford.
By firing high-energy, charged particles (from radioactive decays) at a very thin sheet of gold foil, Rutherford fully expected that all the particles would pass through. And most of them did, but a few spectacularly bounced back! As Rutherford recounted:
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.
What Rutherford discovered was the atomic nucleus, containing virtually all the mass of an atom, confined to a volume one-quadrillionth (10-15) the size of the entire thing. It was the birth of modern physics, and it paved the way for the quantum revolution of the 20th century.
Subscribe to:
Comments (Atom)
-
The Universe began with a bang, but that discovery was a complete accident . In the 1940s, George Gamow and his collaborators put forth a ...
-
Creative Ways to Use Your Interactive Whiteboard After years of interactive whiteboards being touted as the next best thing for engaging...